Equation of State Models Reference
The Equation of State models compute the density with respect to temperature and pressure.
Simcenter STAR-CCM+ provides the following models:
- Constant Density (for gases, liquids, and solids)—based on the assumption that density is invariant throughout the continuum.
- Ideal Gas (for gases)—uses the ideal gas law to express density as a function of temperature and pressure.
- IAPWS-IF97 (Water) (for water)—for information on this model and the companion model for steam, see the IAPWS-IF97 models under Real Gas Models Reference.
- Polynomial Density (for gases, liquids, and solids)—works on the assumption that the density is a function of temperature only.
- Real Gas (for gases)—represents a family of models for situations outside the scope of the ideal gas law. See Real Gas Models Reference
- Thermal Non-Equilibrium Ideal Gas (for gases)—for use at high temperatures and low densities, where the vibrational/electronic energy modes become active yet the density is low enough that equilibration does not occur. See also Thermal Non-Equilibrium Model Reference.
- User Defined EOS (for gases, liquids, and solids)—when the other Equation of State models do not adequately describe your working fluid, this model allows you to specify the density and density derivatives using some user-defined expressions or tables of property data. When you specify thermodynamic properties such as density and enthalpy using tabular input data, Simcenter STAR-CCM+ can evaluate the necessary derivative terms with respect to pressure and temperature by numerically differentiating the tabular data. This calculation eliminates the need to provide more columns of data for the derivative terms. To increase accuracy and robustness of the solver, this derivative data can also be specified explicitly using tabular input data. When explicit derivative values are available, it is best to provide this derivative data to Simcenter STAR-CCM+.
| Model Names and Abbreviations | Constant Density | CD | |
| Ideal Gas | Ideal | ||
| IAPWS-IF97 (Water) | IAPWS-w | ||
| Polynomial Density | PolyD | ||
| Real Gas | Real | ||
| Thermal Non-Equilibrium Ideal Gas | TNEq | ||
| User Defined EOS | UDEOS | ||
| Theory | |||
| Provided by | |||
| Example Node Path | |||
| Requires |
|
||
| Properties | See Equation of State Properties. | ||
| Activates | Physics Models | For CD,
Optional Models:
Boussinesq Model For Ideal, IAPWS-w, PolyD, Real, and TNEq, Energy: Coupled Energy For TNEq, Thermal Non-Equilibrium: Thermal Non-Equilibrium. See Thermal Non-Equilibrium Model Reference. For Real, Real Gas Equation of State box. See Real Gas Models Reference. |
|
| Model Controls (child nodes) |
See Model Controls. |
||
| Materials | See Material Properties. | ||
| Monitors |
|
||
| Field Functions | See Field Functions. | ||
Equation of State Properties
The following table shows which properties are available for which equation of state model:
| CD | Ideal | IAPWS-w | PolyD | Real | TNEq | UDEOS | |
|---|---|---|---|---|---|---|---|
| Compressible | ✓ | ||||||
| Density Limiting | ✓ | ✓ | |||||
| Incompressible | ✓ | ✓ |
See Real Gas Models for the properties of individual real gas models.
- Compressible
- When On, this property specifies that the material is compressible (dependent on working pressure ).
- Density Limiting
- When On, this property activates the Density Limits option that is used to prevent negative densities.
- Incompressible
- When On, this property specifies that the gas is incompressible (not dependent on working pressure ).
Material Properties
The following table shows which material properties become available for which equation of state model:
| CD | Ideal | IAPWS-w | PolyD | Real | TNEq | UDEOS | |
|---|---|---|---|---|---|---|---|
| Critical Pressure | ✓ | ||||||
| Critical Temperature | ✓ | ||||||
| Density | ✓ | ✓ | |||||
| Density Mass-Fraction Derivative | ✓ (if the Multi-Component Gas Model or the Multi-Component Liquid model is activated) |
||||||
| Density Pressure Derivative | ✓ (if Compressible is On) |
||||||
| Density Temperature Derivative | ✓ (if an Energy Model is activated) |
||||||
| Enthalpy | ✓ (if an Energy Model is activated) |
||||||
| Enthalpy Pressure Derivative | ✓ (if an Energy Model is activated and Enthalpy is set to Table, h(T,p)) |
||||||
| Entropy | ✓ (if Compressible is On and an Energy Model is activated) |
||||||
| Molecular Weight | ✓ | ✓ | ✓ | ✓ (if Compressible is On) |
|||
| Molecule Type | ✓ | ||||||
| Polynomial Density | ✓ | ||||||
| Saturation Pressure | ✓ | ||||||
| Saturation Temperature | ✓ | ||||||
| Specific Heat | ✓
(if an Energy Model is activated) |
✓ | ✓ | ✓ |
✓ (if an Energy Model is activated) |
||
| Speed of Sound | ✓ |
✓ (if Compressible is On) |
|||||
| Vibrational-Electronic Specific Heat | ✓ |
See Real Gas Models for the material properties of individual real gas models.
- Critical Pressure
- Displays the model constant (read-only).
- Critical Temperature
- Displays the model constant (read-only).
- Density
- Specifies the density of the fluid .
- Density Mass Fraction Derivative
- Specifies the partial derivative of the user-defined density with respect to mass-fraction for each mixture component .
- Density Pressure Derivative
- Specifies the partial derivative of the user-defined density with respect to pressure .
- Density Temperature Derivative
- Specifies the partial derivative of the user-defined density with respect to temperature .
- Enthalpy
- Specifies the enthalpy of the fluid using one of the following methods:
- Enthalpy Pressure Derivative
- Specifies the partial derivative of the user-defined enthalpy with respect to pressure .
- Entropy
- Specifies the entropy of the fluid using one of the following methods:
- Molecular Weight
- Specifies the molecular weight .
- Molecule Type
- Describes the molecular structure using the following values:
- Polynomial Density
- Specifies the density using a polynomial in temperature, see Using Polynomial in T.
- Saturation Pressure
- Specifies the saturation pressure.
- Saturation Temperature
- Specifies the temperature for the corresponding saturation pressure at which the liquid boils into its vapor phase.
- Specific Heat
- Specifies the fluid-specific heat capacity using one of the following methods:
- Speed of Sound
- Vibrational-Electronic Specific Heat
- Specifies the vibrational-electronic specific heat using one of the following methods:
Model Controls
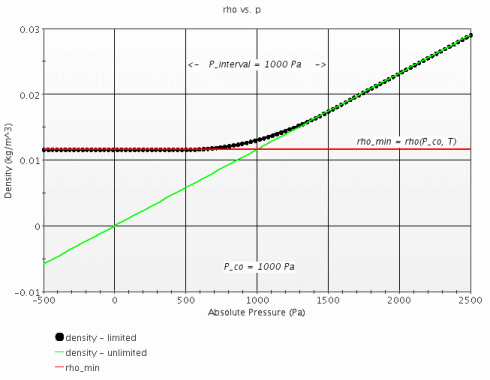
- Density Limits
- When the Density Limiting property is On, specifies the following density limits parameters that allow you to prevent negative densities:
Field Functions
The following table shows which field functions become available for which equation of state model:
| CD | Ideal | IAPWS-w | PolyD | Real | TNEq | UDEOS | |
|---|---|---|---|---|---|---|---|
| Compressibility Factor | ✓ | ||||||
| Critical Pressure | ✓ | ||||||
| Critical Temperature | ✓ | ||||||
| Enthalpy of [material] | ✓ | ||||||
| Entropy | ✓ | ✓ | ✓ | ||||
| Entropy Function | ✓ | ✓ | |||||
| Gas Constant | ✓ | ✓ | ✓ | ||||
| IAPWS Region ID | ✓ | ||||||
| Mach Number | ✓ | ✓ | ✓ | ||||
| Molecular Weight | ✓ | ✓ | ✓ | ||||
| Ratio of Specific Heats | ✓ | ✓ | ✓ | ||||
| Reduced Pressure | ✓ | ||||||
| Reduced Temperature | ✓ | ||||||
| Relative Mach Number | ✓ | ✓ | ✓ | ||||
| Saturation Pressure | ✓ | ||||||
| Saturation Temperature | ✓ | ||||||
| Speed of Sound | ✓ | ✓ | ✓ |
See Real Gas Models for the field functions of individual real gas models.
- 可压缩性因子
- 表示实际气体与理想气体的偏差量,表示为
。通常,此因子取值大约为 1。
对于平衡空气模型,可压缩性因子表示离解度。这并非理想行为,但值的变化范围更广(大约介于 1 和 6 之间)。Eqn. (686) 中给出了平衡空气模型的简化公式。
- 临界压力
- 表示为 。
- 临界温度
- 表示为 。
- [材料] 的焓
- 指定材料的焓。
- Entropy
- Entropy Function
- The entropy function is calculated as:
- 气体常数
- 单位气体常数。
- IAPWS 区域 ID
- Simcenter STAR-CCM+ 针对特定的 IAPWS 有效区域计算的数据。
- 马赫数
- 局部马赫数。
- 分子量
- 为材料指定的分子量。
- 比热比
- 表示为:
其中 为比热 J/(kg K), 为单位气体常数 J/(kg K)。
- 折算压力
- 表示为 。
- 折算温度
- 表示为 。
- 相对马赫数
- 饱和压力
- 为材料指定的饱和压力。
- 饱和温度
- 为材料指定的饱和温度。
- 声速
- 介质中的声音传播速率。