Modeling Electrochemical Surface Reactions
Simcenter STAR-CCM+ allows you to model electrochemical reactions on boundary surfaces around the physics continuum for the fluid, on the interface surfaces between a solid continuum and the fluid, and on unresolved surfaces inside of porous media. In all of these cases, except in fluid films, you can choose to model the production and consumption of electrochemical species from reactions—or you can choose to model the production and consumption of charged species from reactions on boundaries and in porous regions.
Follow this procedure to model electrochemical surface reactions.
Refer to Electrochemical Species Model Reference and Electrochemical Reaction Model Reference.
| 注 | For improved convergence, use a double precision version of Simcenter STAR-CCM+. |
-
Set up the fluid physics continua.
- If the Gradients and Electrochemical Species models are both selected and any electrochemical species are in low concentration, select the node and set Limiter Method to MinMod.
- To neglect diffusion at flow boundaries, within the fluid continuum, multi-select all segregated flow, species, and energy models and deactivate Flow Boundary Diffusion.
-
If the simulation includes a solid on which surface reactions take place, add a physics continuum for this solid.
-
If you are using the Multiphase model, set up the phases
and define any phase interactions. See Defining Eulerian Phases and Defining Phase
Interactions.
For Eulerian phases which participate in reactions, select the Multi-Component Liquid or Multi-Component Gas model.
-
If you have a solid continuum present in the simulation, you can optionally choose to solve for the electric potential within the solid material. Otherwise, set a constant electric potential value on the surface that bounds the fluid:
- To set a constant electric potential on the fluid bounding surface:
- Expand the node.
- Select the node and set Mechanism to [Electrochemical Mechanism].
- Select the node and specify the electrode electric potential value.
- To account for the electric potential variation
within the fluid and the solid at a reacting interface, select the node and set Mechanism to
[Electrochemical Mechanism].
注 In contrast to using an electrochemical surface mechanism at boundaries, there is no option to set the Electrode Electric Potential within the interface physics values. The Electric Potential value for the electrode is obtained internally from the solid.
- To set a constant electric potential on the fluid bounding surface:
This feature is also useful when modeling some applications in which electrochemical species are defined, such as catalyst resistance in fuel cells, corrosion products or organic tissues at interfaces, and SEI interfaces in batteries.
-
To account for electrical resistance, do one of the following:
- When an electrochemical surface mechanism is set on the interface, specify the electrical resistance at the interface. Select the node and specify the electrical resistance. When more than one fluid region contacts the solid region, you can specify the electrical resistance at each boundary or interface independently. This option is not available for baffle interfaces between porous phases when using the Electrodynamic Potential model.
- When an electrochemical surface mechanism is set on the boundary, specify the electrical resistance at the boundary. Select the node and specify the electrical resistance.
- When an electrochemical surface mechanism is set on the region, specify the electrochemical resistance at the region. Select the node and specify the electrical resistance.
This feature is useful when using phasic porous media to simulate reactive catalyst layers in fuel cells.
-
To account for thermal resistance:
- Select the node and set Baffle Thermal Option to Conducting.
- Select the node and specify the thermal resistance.
-
If the simulation uses the Porous Media model to resolve
electrochemical reactions on geometrically unresolved surfaces, set up the
porous phases.
- Define the material species.
-
If the Electrochemical Species model is selected, create
the solid species that represent the solid surface in the electrochemical
surface reactions:
- Expand the node.
- Right-click the node and select Select Mixture Components.
- Select all of the solid species that represent the solid surface in the electrochemical surface reactions. If you cannot find a solid species component, you can select a similar solid species component and modify its material properties to change the type and quantity of atoms.
- Click Apply, then Close.
If you did not select the Electrochemical Species model, the solid material properties are taken from the solid physics continuum.
-
To modify the Electrochemical Species model, do the
following:
- To specify the solid ion electrochemical species components, in the solid continuum, right-click the node and select Select Mixture Components. You can add and amend species in the same way as described above for the Electrochemical Species model.
- Expand the node, select the Electrical Conductivity node, and set Method to Electrochemical Species.
- There are the same number of each type of atom in the reactants and products—in any reaction, matter cannot be created or destroyed.
- The overall charge from the reactants and products must balance so that the total electric charge that is created in each reaction is zero.
- Electrons are specified as reactants or products—in electrochemical reactions, electrons must be produced or consumed to generate an electric current.
- Expand the node then right-click the Surface Mechanism Manager node and select New Surface Mechanism.
- If you want to account for non-default phase volume fraction in sorption and electrochemistry reactions, select the node and activate Expert options. This option enables Phase Interaction Area Exponents where you can set reaction exponents, see Phase Interaction Area Exponents.
-
Create the reactions and specify the reaction type for each reaction.
- Right-click the node and select New Reaction.
- For each reaction, select the node and set Type.
See Type. -
Set the reaction properties such as equilibrium potential, reaction
formulation, exchange current, and/or vacancy rate exponent (only available with
the Sub-grid Particle Intercalation model). See, Reaction [n] Reference for a full list of
available properties and set-up options.
- For tertiary current distribution type reactions
(when species are resolved in the simulation): The solid, electrically
conducting phase will be detected via the continuum of the electron that is
added to the reaction.
The electrolyte phase is detected by the electrochemical species type reactants, which are only allowed to be added from one solid physics continuum or one solid phase. This continuum needs to have both the Electrodynamic Potential model, and the Solid Ion model selected.
- For secondary current distribution type porous media reactions (when no multi-component, electrochemical species, or solid ion model is selected in the simulation), the node provides a Secondary Current Phase Interaction Option.
-
If applicable, continue to specify reactants and products.
Since electrons can exist in different continua, take care to select electrons from the correct location.When using the Porous Media model, you select the electrons from the solid porous phase that is in the same continuum as the reaction (this defines the porous phase as the conductor or binder), and electrochemical species from the Solid Ion model in the interfacing continuum or ions from the Multi-Component Liquid when the Concentrated Electrolyte Model is present (this defines the interfacing continuum as the electrolyte).
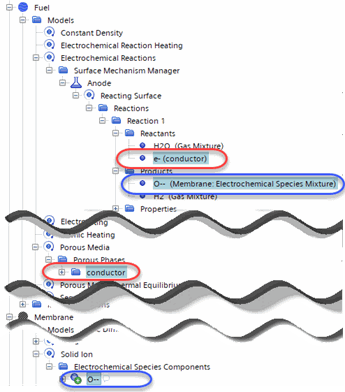
- Right-click the node and select a reactant. Repeat this step until all reactants are selected.
- Right-click the node and select a product. Repeat this step until all products are selected.
-
Define the surface/region upon which the mechanism is applied.
Surface Type Interface (recommended when using the Porous Media model)
Select the node and set Mechanism to the [Electrochemical Mechanism] that is required. Boundary Select the node and set Mechanism to the [Electrochemical Mechanism] that is required. Region (for homogenized, that is distributed, non-resolved interfaces)
Select the node and set Mechanism to the [Electrochemical Mechanism] that is required. - If you choose to work with non-default values of Phase Interaction Area Exponents, select the Phase Interaction Area Exponents node and set Phase exponent values, see Phase Interaction Area Exponents. Typical values for Phase Interaction Area Exponents range between 0.3 to 3. Setting the exponent value to zero makes the chemical reaction independent from the phase volume fraction changes.
-
Finalize any remaining settings for the region types and the conditions at the
regions, boundaries, or interfaces.
When using the Electrochemical Species model, porous baffle interfaces are not allowed.
-
To define initial conditions for the electric potential, do one of the
following:
- If you want to specify the electric potential manually, select the node and set the values for the electric potential.
Simcenter STAR-CCM+ uses values that you set for the electric potential initial conditions everywhere.
- If you want to let Simcenter STAR-CCM+ automatically initialize the electric potential, select the node and set Method to Presolve. Prior to running the electric potential presolver, Simcenter STAR-CCM+ initializes the electric potential using a parts-based or region-based approach—dependent upon the set-up. For more information, see Expert Initialization.
- If you want to specify the electric potential manually, select the node and set the values for the electric potential.
-
Set up Scenes and Plots to visualize the solution.
For example, you can visualize the following field functions, among others, in a scalar scene or plot them on an x-y plot:
- charged species mobility of specific electrochemical species
- electric current density
- electric potential
- electrochemical reaction rate of a specific electrochemical reaction mechanism
- migration flux of specific electrochemical species
- molar concentration of specific electrochemical species
- number density of specific electrochemical species
- molecular diffusivity of specific electrochemical species
- bulk substance electrochemical reaction flux of a porous phase
- surface/average molar concentration
注 To check energy conservation, add reports for electric power flux and energy loss then create monitors and a single plot from these reports. For more information, see the Electrochemistry: Solid Oxide Fuel Cell Tutorial. - Run the simulation.