Electrochemical Surface Reactions
When modeling electrochemistry, Simcenter STAR-CCM+ provides the Electrochemical Reaction model which allows you to model charge transfer reactions at electrode surfaces.
In electrochemistry simulations, electrochemical reactions take place at surfaces, where electrons are consumed or produced. The rate of reaction depends on the concentrations of reactants and products as well as the potential difference across the surface.
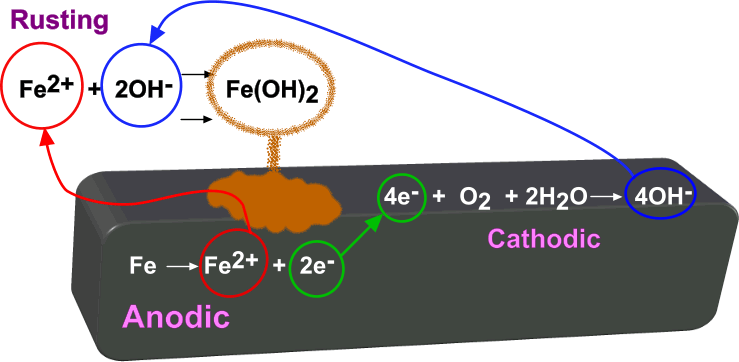
Electrochemical reactions that lose electrons are known as oxidation or anodic reactions. Reactions that gain electrons are known as reduction or cathodic reactions. Often these reactions occur together and the electrons that the anodic reactions create, drive the cathodic reactions.
The following example shows the electrochemical reactions that occur within, and surrounding, an iron surface that is in contact with water and oxygen.
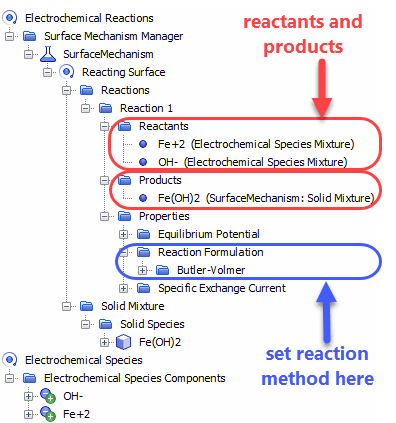
When using the Electrochemistry Reacting model with the Electrochemical Species model, you can specify reaction methods and create mechanisms for electrochemical surface reactions which include reactant and product species that you specify. Specifying species allows you to include the effects of the ion concentrations on reactions. You can choose a coupled or segregated approach to solve for electrochemical species concentrations.
However, if you are only concerned with electric currents and not concerned with the effects of the ion concentrations on reactions, you can model the same reaction excluding the Electrochemical Species model. Excluding the Electrochemical Species model allows you to create electrochemical surface mechanisms in which you specify surface reactions without specifying the reactant and product species.
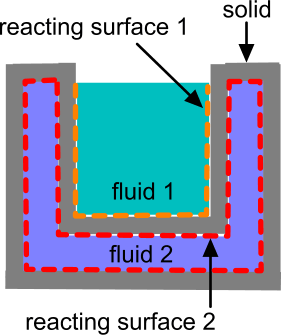
This feature is compatible with solid shell regions, fluid films, and porous phases. However, you can only define reactions at interfaces between solid and fluid physics continua, you cannot define the reacting surface interface between two solids. See Modeling Electrochemical Surface Reactions.
Simcenter STAR-CCM+ uses a linear least squares based method to automatically initialize the electric potential throughout all parts (or regions) in which the electrochemical reactions model is active—and any corresponding electrically connected parts (or regions).
Sorption
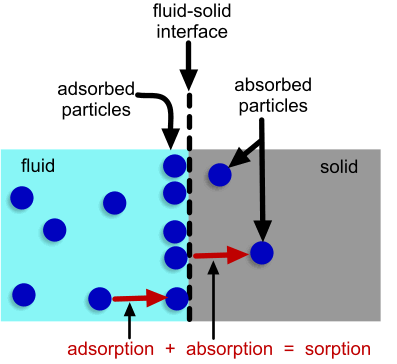
Although sorption is not truly a type of electrochemical reaction (electrons are neither consumed or produced), the process of sorption is important in some electrochemical applications. Sorption can be described as a combination of adsorption and absorption of particles. Particles from a multi-component fluid adhere to the surface of a solid (adsorption) and then the particles move into the solid (absorption).
- a fluid continuum which uses the electrochemical reactions model with the multi-component liquid or multi-component gas model
- a phase which uses the Eulerian Multiphase model with the electrochemical reactions model, and the multi-component liquid or multi-component gas model
- a phase which uses the Mixture Multiphase model with the electrochemical reactions model, and the multi-component liquid or multi-component gas model
When the particles enter the solid continuum, they can participate in any electrochemical reactions that are defined within that solid continuum. Simulating sorption may be required when simulating some types of fuel cells—for example, Polymer Electrolyte Membrane (PEM) fuel cells.
To model sorption, follow the workflow in Modeling Electrochemical Surface Reactions.